Abstract
Introduction: The optimum number of cycles and dose of Cytarabine consolidation in older patients (age 60 and above) with AML in first remission remains unclear.
Methods: We retrospectively reviewed electronic medical records of patients. We identified 81 patients that were diagnosed with AML ( at age 60 and above) and received induction chemotherapy followed by Cytarabine consolidation between 2008 and 2017.
Results: We reviewed the outcomes of 81 patients and report overall survival (OS) and relapse free survival (RFS) for the 69 patients reaching a landmark of 120 days from diagnosis without relapse or death (to exclude patients who would not be amenable to extended consolidation). Median follow up was 19.4 months (range 4.2-105.7). The median age at diagnosis was 65 (range 60-77); 86% were White. The majority were men (66%) ; 60% received daunorubicin-based induction [dose range 45 mg - 90 mg/m2] and 19% received re-induction chemotherapy.
The cytogenetics based on ELN 2017 criteria were 71% intermediate, 13% favorable,10% adverse and for 6% not available (N/A.) From the 69 patients 16 were documented to be FLT3 ITD positive and 17 NPM1 positive.
Twenty out of 69 patients (29%) received allogeneic hematopoietic cell transplantation (alloHCT). From those transplanted 5 patients were transplanted at CR2, one patient with relapse disease and the rest at CR1.
Thirty patients relapsed and 27 patients died. At time of last follow up or death 56% of patients were on CR1, 13% on CR2 while the rest had relapsed disease [up to 3 relapses].
The median number of Cytarabine consolidation cycles was 2 (range 1-4 cycles) with Cytarabine total dose per cycle ranging between 6 gr/m2-18 gr/m2. The median cumulative dose from all cycles of consolidation was 18 gr/m2. We considered a cumulative dose ≤18 gr/m2 to be "low intensity (LI)" and > 18 gr/m2 "high intensity (HI)".
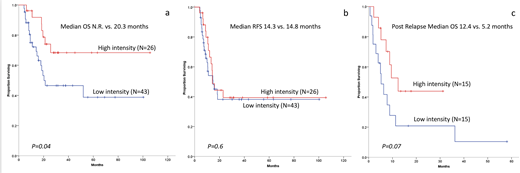
OS was superior for HI patients [(Median not reached vs. 20.3 months, P=0.04), Figure 1a]. However there was no difference in RFS between groups [ median 14.3 Vs 14.8 months, p=0.6, Figure 1b]. When data were censored at the time of alloHCT, the OS advantage for HI was no longer statistically significant (p=0.07). In multivariate analysis for OS HI consolidation, intermediate and favorable risk cytogenetics had a favorable impact. Black race was associated with inferior OS. Since HI was associated with improved OS but not RFS we hypothesized that HI had better post relapse survival. In fact, there was a trend towards better post relapse survival among HI patients (N=15) compared to LI patients (N=15) [( median 12.4 vs. 5.2 months, p=0.070), Figure 1c].
Conclusion: The intensity of Cytarabine consolidation for older AML patients in CR1 was associated with improved OS but not RFS. Although the selection of LI or HI by the treating physician did not affect risk of relapse, our data suggest that HI consolidation is likely a surrogate for factors that make patients more amenable to successful post relapse therapy.
Costa:Amgen: Honoraria, Research Funding; Abbvie: Research Funding; Janssen: Research Funding; Karyopharm: Research Funding; BMS: Research Funding; Celgene: Honoraria, Research Funding; Sanofi: Honoraria. Erba:Janssen: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; MacroGenics: Consultancy; Seattle Genetics: Consultancy, Research Funding; Astellas: Research Funding; Novartis: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Juno: Research Funding; Immunogen: Consultancy, Research Funding; Takeda/Millenium: Research Funding; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Seattle Genetics: Consultancy, Research Funding; MacroGenics: Consultancy; MacroGenics: Consultancy; Celgene: Consultancy, Speakers Bureau; Agios: Consultancy, Speakers Bureau; Astellas: Research Funding; Juno: Research Funding; Seattle Genetics: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Takeda/Millenium: Research Funding; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Jazz: Consultancy, Speakers Bureau; Janssen: Research Funding; Jazz: Consultancy, Speakers Bureau; Immunogen: Consultancy, Research Funding; Pfizer: Consultancy, Other: grant; Novartis: Consultancy, Speakers Bureau; Takeda/Millenium: Research Funding; Seattle Genetics: Consultancy, Research Funding; Pfizer: Consultancy, Other: grant; Agios: Consultancy, Speakers Bureau; Amgen: Research Funding; Agios: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Amgen: Research Funding; Incyte: Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Immunogen: Consultancy, Research Funding; Novartis: Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Pfizer: Consultancy, Other: grant; Immunogen: Consultancy, Research Funding; Pfizer: Consultancy, Other: grant; Celgene: Consultancy, Speakers Bureau; Takeda/Millenium: Research Funding; MacroGenics: Consultancy; Juno: Research Funding; Juno: Research Funding; Agios: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Celgene: Consultancy, Speakers Bureau; Daiichi Sankyo: Consultancy, Research Funding; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Daiichi Sankyo: Consultancy, Research Funding. Papadantonakis:Agios pharmaceuticals: Honoraria, Other: advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal